Draw The Structure Of Cyclohexane
3.11: Cyclohexane
-
- Last updated
- Save as PDF
- Page ID
- 28136
Objectives
After completing this section, you should be able to
- explain why cyclohexane rings are free of angular strain.
- draw the structure of a cyclohexane ring in the chair conformation.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- chair conformation
- twist-boat conformation
We will find that cyclohexanes tend to have the least angle strain and consequently are the most common cycloalkanes found in nature. A wide variety of compounds including, hormones, pharmaceuticals, and flavoring agents have substituted cyclohexane rings.
testosterone, which contains three cyclohexane rings and one cyclopentane ring
Rings larger than cyclopentane would have angle strain if they were planar. However, this strain, together with the eclipsing strain inherent in a planar structure, can be relieved by puckering the ring. Cyclohexane is a good example of a carbocyclic system that virtually eliminates eclipsing and angle strain by adopting non-planar conformations. Cycloheptane and cyclooctane have greater strain than cyclohexane, in large part due to transannular crowding (steric hindrance by groups on opposite sides of the ring). Cyclohexane has the possibility of forming multiple conformations each of which have structural differences which lead to different amounts of ring strain.
| | | | |
Conformations of Cyclohexane
A planar structure for cyclohexane is clearly improbable. The bond angles would necessarily be 120º, 10.5º larger than the ideal tetrahedral angle. Also, every carbon-hydrogen bond in such a structure would be eclipsed. The resulting angle and eclipsing strains would severely destabilize this structure. The ring strain of planar cyclohexane is in excess of 84 kJ/mol so it rarely discussed other than in theory.
Cyclohexane in the strained planar configuration showing how the hydrogens become eclipsed.
Chair Conformation of Cyclohexane
The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. If two carbon atoms on opposite sides of the six-membered ring are bent out of the plane of the ring, a shape is formed that resembles a reclining beach chair. This chair conformation is the lowest energy conformation for cyclohexane with an overall ring strain of 0 kJ/mol. In this conformation, the carbon-carbon ring bonds are able to assume bonding angles of ~111o which is very near the optimal tetrahedral 109.5o so angle strain has been eliminated.
Also, the C-H ring bonds are staggered so torsional strain has also been eliminated. This is clearly seen when looking at a Newman projection of chair cyclohexane sighted down the two central C-C bonds.
Newman projection of cyclohexane
Interactive Element
The 3D Structure of Chair Cyclohexane
How to Draw the Chair Conformation
Boat Conformation of Cyclohexane
The Boat Conformation of cyclohexane is created when two carbon atoms on opposite sides of the six-membered ring are both lifted up out of the plane of the ring creating a shape which slightly resembles a boat. The boat conformation is less stable than the chair form for two major reasons. The boat conformation has unfavorable steric interactions between a pair of 1,4 hydrogens (the so-called "flagpole" hydrogens) that are forced to be very close together . This steric hindrance creates a repulsion energy of about 12 kJ/mol. An additional cause of the higher energy of the boat conformation is that adjacent hydrogen atoms on the 'bottom of the boat' are forced into eclipsed positions. For these reasons, the boat conformation about 30 kJ/mol less stable than the chair conformation.
A boat structure of cyclohexane (the interfering "flagpole" hydrogens are shown in red)
Twist-Boat Conformation of Cyclohexane
The boat form is quite flexible and by twisting it at the bottom created the twist-boat conformer. This conformation reduces the strain which characterized the boat conformer. The flagpole hydrogens move farther apart (the carbons they are attached to are shifted in opposite directions, one forward and one back) and the eight hydrogens along the sides become largely but not completely staggered. Though more stable than the boat conformation, the twist-boat (sometimes skew-boat) conformation is roughly 23 kJ/mol less stable than the chair conformation.
A twist-boat structure of cyclohexane
Half Chair Conformation of Cyclohexane
Cyclohexane can obtain a partially plane conformation called "half chair" but with only with excessive amounts of ring strain. The half chair conformation is formed by taking planar cyclohexane and lifting one carbon out of the plane of the ring. The half chair conformation has much of the same strain effects predicted by the fully planar cyclohexane. In the planar portion of half chair cyclohexane the C-C bond angles are forced to 120o which creates significant amounts of angle strain. Also, the corresponding C-H bonds are fully eclipsed which create torsional strain. The out-of-plane carbon allows for some of the ring's bond angles to reach 109.5o and for some of C-H bonds to not be fully eclipsed. Overall, the half chair conformation is roughly 45 kJ/mol less stable than the chair conformation.
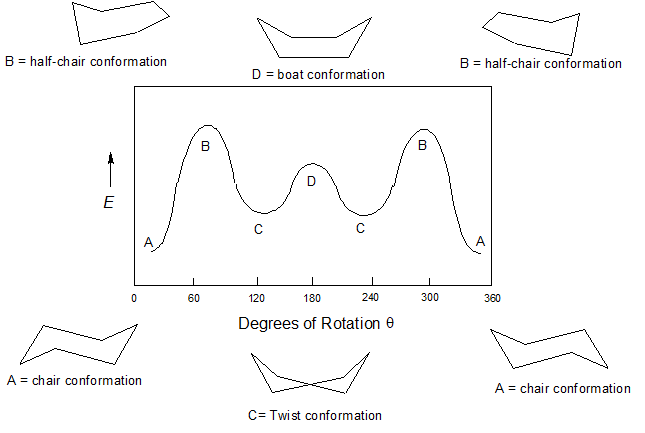
Conformation Changes in Cyclohexane - "Ring Flips"
Cyclohexane is rapidly rotating between the two most stable conformations known as the chair conformations in what is called the "ring flip" shown below. The importance of the ring flip will be discussed in the next section.
"Ring flip" describes the rapid equilibrium of cyclohexane rings between the two chair conformations
It is important to note that one chair does not immediately become the other chair, rather the ring must travel through the higher energy conformations as transitions. At room temperature the energy barrier created by the half chair conformation is easily overcome allowing for equilibration between the two chair conformation on the order of 80,000 times per second. Although cyclohexane is continually converting between these different conformations, the stability of the chair conformation causes it to comprises more than 99.9% of the equilibrium mixture at room temperature.
Exercises
1) Consider the conformations of cyclohexane: half chair, chair, boat, twist boat. Order them in increasing ring strain in the molecule.
Solutions
1) Chair < Twist Boat < Boat < half chair (most ring strain)
Draw The Structure Of Cyclohexane
Source: https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/UIS%3A_CHE_267_-_Organic_Chemistry_I_(Morsch)/Chapters/Chapter_04%3A_Alkanes/3.11%3A_Cyclohexane
Posted by: epleymisibromes.blogspot.com

0 Response to "Draw The Structure Of Cyclohexane"
Post a Comment